
Bacterial protection against C diff
Targeted bacterial restoration of colonization resistance against C. difficile in the gastrointestinal tract.
Clostridium difficile infection (CDI) is the leading cause of antibiotic-associated colitis and is responsible for significant morbidity, mortality and increased healthcare costs. Antibiotics disrupt the indigenous gut microbiota, reducing resistance to C. difficile colonization. Our knowledge of the mechanism(s) by which the gut microbiota confers resistance to CDI is incomplete, presenting a significant roadblock to improving preventative and therapeutic approaches against this pathogen. My long-term goal is to understand how the gastrointestinal tract microbiota mediates colonization resistance against enteric pathogens, including C. difficile. The overall objective of this application is to define members of the gut microbiota that are able to alter bile acids and consume sugars which are required for C. difficile colonization and pathogenesis. Based on preliminary studies the central hypothesis is that the production and consumption of specific metabolites (secondary bile acids and sugars) by the indigenous gut microbiota contribute to colonization resistance against C. difficile. The rationale that underlies the proposed research is that the targeting of metabolites required for C. difficile colonization has the potential to improve directed therapeutic approaches for this infection. Guided by strong preliminary data, this hypothesis will be tested by exploring the following key questions: 1) Can restoring microbial-mediated secondary bile acid metabolism in the large intestine restore colonization resistance against C. difficile? and 2) Can restoring bacteria that are able to compete for the same nutrients (sugars) as C. difficile requires for growth reestablish colonization resistance against C. difficile?
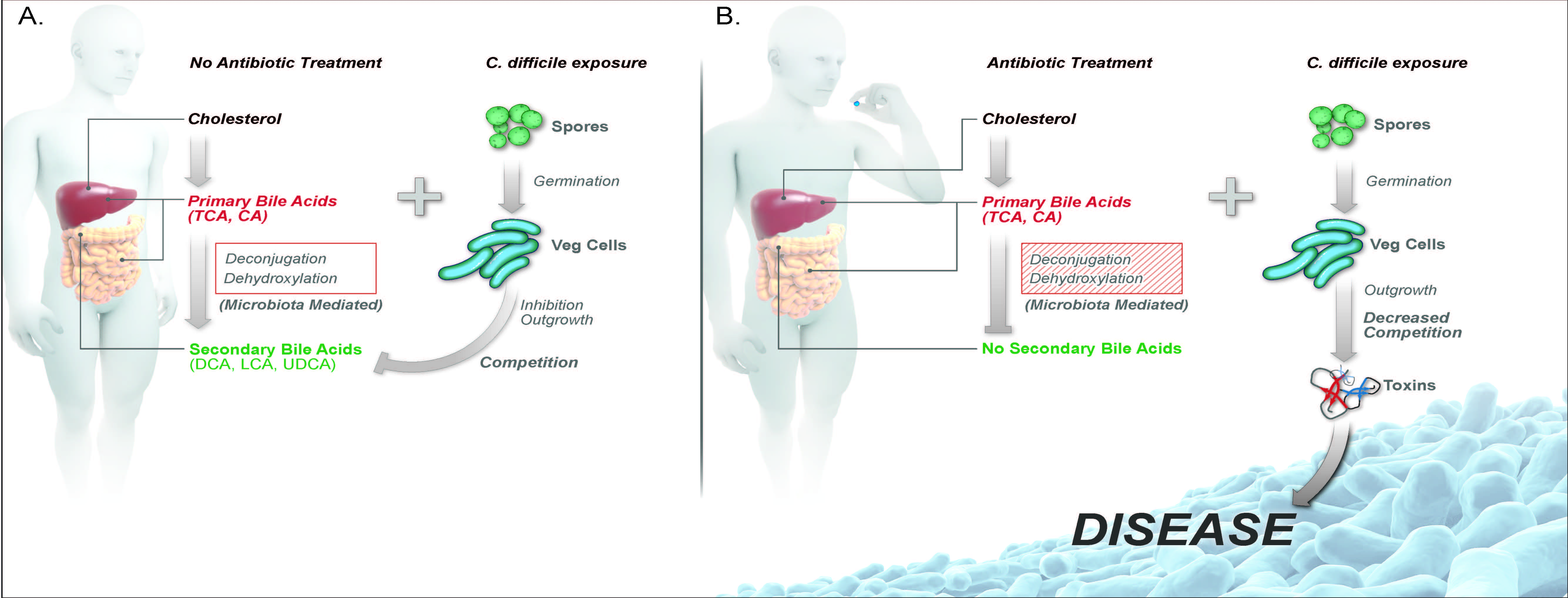
Antibiotic-induced alterations in gut microbial metabolism decreases colonization resistance against C. difficile. A) Bile acids are synthesized from cholesterol by hepatic enzymes. Once synthesized in the liver, primary bile acids (ex. conjugated, TCA, and unconjugated, CA) travel through the small intestine, where 95% of bile is absorbed in the terminal ileum and through the hepatic system. The small amount of bile acids that reaches the large intestine are further biotransformed by members of the gut microbiota via deconjugation and dehydroxylation into secondary bile acids, including DCA, LCA, and UDCA. C. difficile spores can use primary bile acids TCA and CA in the ileum for germination from a spore to an actively growing vegetative cell. The presence of secondary bile acids and competition of nutrients from other members of the indigenous gut microbiota are able to inhibit C. difficile outgrowth and colonization in the large intestine. B) Antibiotic treatment alters the gut microbiota structure, specifically decreasing bacteria that are able to deconjugate and dehydroxlate primary bile acids into secondary bile acids. The loss of secondary bile acid metabolism and competition from the gut microbiota allows for C. difficile outgrowth, toxin production and disease. Abbreviations: TCA, taurocholate; CA, cholate; DCA, deoxycholate; LCA, lithocholate; and UDCA, ursodeoxycholate. Theriot and Young. Annual Rev Microbiology October 2015.
